Aluminium Hydroxide Weak or Strong Base
Since it is composed of the hydroxide anion OH- it is a strong baseIn solution the hydroxide anion will completely react with any available protons that is why KOH is a strong baseIt is not an acid of any type weak or strong since KOH does not contribute any protons to solution. A compound is a strong base when it completely dissociates in an aqueous solution and liberates a large number of hydroxide ionsAll moles of the strong base dissociate into hydroxide ionOH and no part remains undissociated into.

Unit 9 Notes Acids Bases And Salts Acids An Acid Is A Substance That Produces Hydrogen Ion H Or Hydronium H 3 O In Solution Ppt Download
3 N H 3 a q H 2 O l N H 4 a q O H a q.

. When measuring the relative H hydroxide concentration in a solution pOH is sometimes used instead of pH. Strong bases have a less dramatic effect on pH than weak bases. LiOH acts as a Lewis base since it has an OH ion that can donate the electron pair to another compound acceptor.
Thus weak acid and base solutions contain multiple charged and uncharged species in dynamic equilibrium. However the reaction is reversible and at any one time about 99 of the ammonia is still present as. To know whether CaOH 2 is a strong base or weak you must know the basic difference between a strong base or a weak base.
KOH is potassium hydroxide. Start studying StrongWeak acidsbases. Answer 1 of 12.
Since it is composed of the hydroxide anion OH- it is a strong base. All hydroxides are bases. Prachi Singh Apr 19 2018 Weak base because it dissociate less OH- ion when dissolve in water Upvote 4.
Since it is composed of the hydroxide anion OH- it is a strong base. Which is a strong base. LiOH is the strong base.
On the flip side the hydroxide in aluminum hydroxide can also act as a weak acid when reacting with the strong base. Lithium hydroxide LiOH is the base since it releases OH ions on dissolving in an aqueous solution. The base dissociation constant K_b is related to the acid dissociation constant in that it mathematically quantifies the relative strength of the base.
Ammonia is a typical weak base. KOH is potassium hydroxide. Is aluminium hydroxide a weak or strong base.
Aluminium hydroxide is a weak base but nitric acid is a strong acid. While ammonia NH 3 is weak base because it accepts protons from water to produce fewer hydroxide ions in solutionBecause strong bases fully dissociate in water they produce lots of hydroxide ions in solution making the solution more basic. Learn vocabulary terms and more with flashcards games and other study tools.
D What is the pH of a 60 M solution of H2SO. E Calculate the pH of a 00036 M solution of oxalic acid that is 050 ionized 3_Indicate whether the following bases are strong or weak a Aluminum hydroxide b Sodium hydroxide c Lithium hydroxide d Ammonium hydroxide e Copper 1 hydroxide f Silver hydroxide g Rubidium hydroxide. Is Copper Hydroxide a.
In solution the hydroxide anion will completely react with any available protons that is why KOH is a strong base. Weak Acid Strong Base Titration. Sodium hydroxide NaOH is strong base because it fully dissociates in water to produce hydroxide ions.
Follow this answer to receive notifications. The salt is formed by reacting strong acid and weak base is known as acidic. See DISSOCIATION CONSTANTS OF INORGANIC ACIDS AND BASES IN AQUEOUS SOLUTION at the page numbered 143 page 12 of the file.
If our subject is a base it will readily accept H ions while if it is an acid it will readily donate H ions. A weak base is a base that partially dissociates or breaks apart in solution. Aluminum hydroxide is insoluble in water and is a weak base.
It is not an acid of any type weak or strong since KOH does not contribute any protons to solution. Manoj Apr 19 2018. It is a weak acid with pKa of 5.
6H2OThe reaction between aluminum hydroxide a strong base and. LiOH acting as Arrhenius base and Bronsted-Lowry base. Lets first look at why ammonium hydroxide is a base at all strong or weak being irrelevant.
Ammonia itself obviously doesnt contain hydroxide ions but it reacts with water to produce ammonium ions and hydroxide ions. Ammonium hydroxide consists of two ions. A strong base typically has ah hydroxide that it can donate toe water.
No it is not a strong base. Sodium hydroxide is soluble in water and is a strong base while aluminum hydroxide is insoluble in water and is a weak base. Aluminium hydroxide is a base.
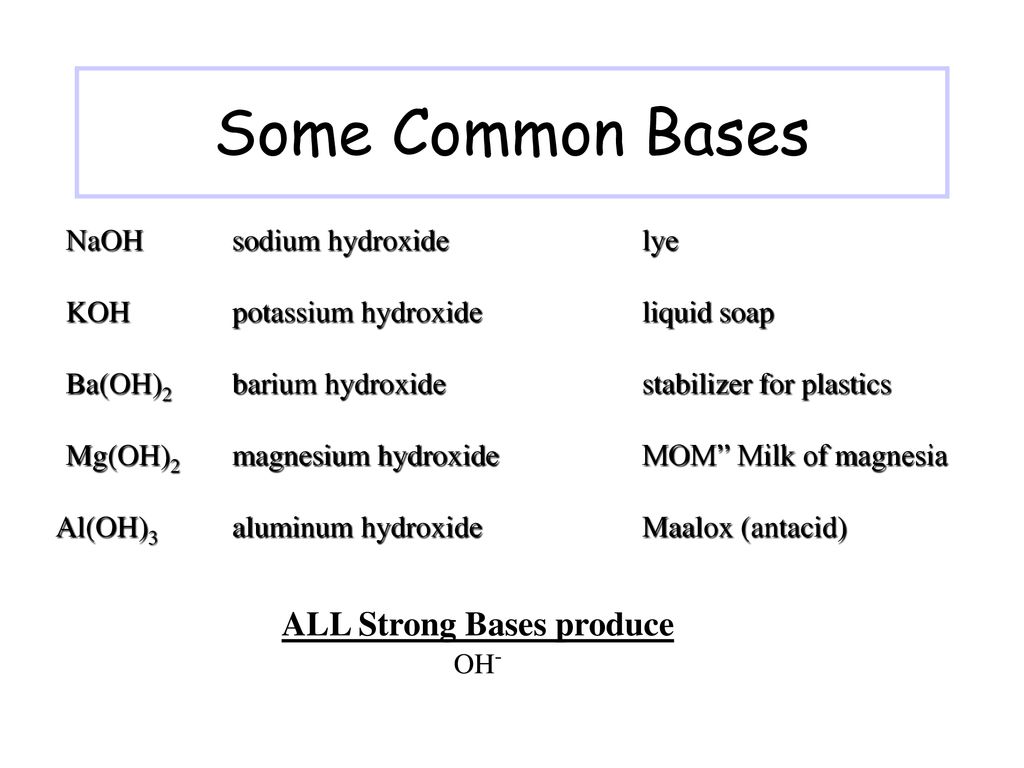
The Chemistry Of Acids And Bases Ppt Download

Acids Bases And Salts Get To Know Them Facts About Acids And Bases An Acid Is A Substance That Produces Hydrogen Ions H A Bases Is A Substance Ppt Download
No comments for "Aluminium Hydroxide Weak or Strong Base"
Post a Comment